How Does CAR T-Cell Therapy Work?
Immunotherapy Program
How is Immunotherapy Different from Conventional Cancer Treatment?
Why Refer/Choose Northside Hospital’s Immunotherapy Program?
Immunotherapy Patients Have Access To:
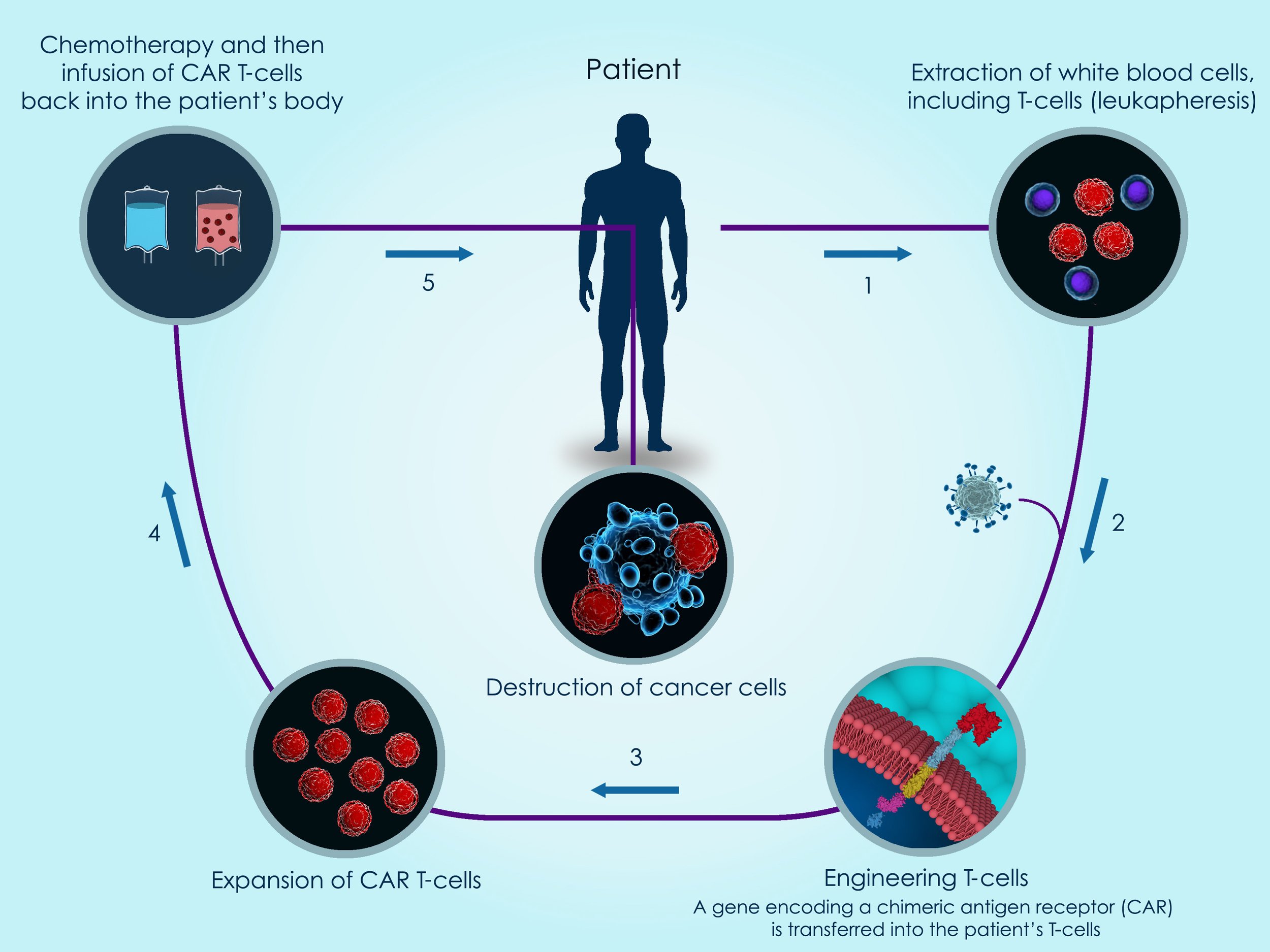
How Does CAR T-Cell Therapy Work?
CAR T-Cell Treatment
Step One: Pre-CAR T-cell testing
To determine if a patient is a CAR T-cell therapy candidate, re-staging radiology studies, an EKG/echocardiogram, a bone marrow biopsy, blood work, and meetings with a BMTGA physician, a clinical research nurse, and a nurse care coordinator will occur. The BMTGA physician will review all test results and determine if a patient is a candidate to receive CAR T-cell therapy.
Step Two: T-cell collection procedure
A central line catheter is placed in a large chest vein and connects to a blood collection machine, which removes T-cells and then returns any remaining blood cells components collected back to the patient via the central line catheter. Leukapheresis/apheresis is a one-day outpatient procedure and is performed at Atlanta Blood Services.
Step Three: Reprogram/Expansion/Manufacturing
Once T-cell collection is completed, the T-cell product is shipped to a CAR T-cell manufacturing laboratory.
Reprogram: T-cells are then genetically engineered to produce special receptors on their surface called Chimeric Antigen Receptors, or CARs. This enables the T-cells to better recognize, kill and destroy cancer cells.
Expansion: The manufacturing laboratory grows and engineers CAR T-cells until they number in the billions. Engineering and growing enough quantities of CAR T-cells may take a few weeks to produce.
Manufacturing: Once the target number of expanded/manufactured CAR T-cells is achieved, the CAR T-cells are then frozen and shipped back to the Northside Hospital Hematopoietic Stem Cell Laboratory.
Step Four: Chemotherapy
Prior to CAR T-cell infusion, a patient receives disease-specific chemotherapy. Chemotherapy creates space within the immune system that allows the infused, reprogrammed CAR T-cells to grow and multiply.
Step Five: CAR T-cell infusion
CAR T-cell infusion occurs on the Northside Hospital inpatient Blood and Marrow Transplant, Leukemia, and Immunotherapy Unit. The infusion process is similar to receiving a blood product infusion. To prevent potential CAR T-cell infusion reactions, pre-medications are given.
Step Six: Target and destroy
CAR T-cells then multiply and, with guidance from their engineered receptors, are able to recognize and then kill cancer cells. During this period, if side effects develop, inpatient hospitalization may be required for several days or weeks.
The most common side effects are:
Fever (100.5°F/38°C or higher)
Difficulty breathing
Chills or shaking chills
Confusion
Dizziness or lightheadedness
Severe nausea, vomiting, or diarrhea
Fast or irregular heartbeat
Severe fatigue or weakness
Step Seven: Post CAR T-cell recovery
Recovery from CAR T-cell therapy can take two to three months. During this time, frequent clinic monitoring visits and possible hospitalizations may occur.
Step Eight: Disease restaging follow-up studies
At days 30 and 100 post-CAR T-cell infusion and at additional time points, disease re-staging studies are performed at The Blood and Marrow Transplant Group of Georgia and Northside Hospital.